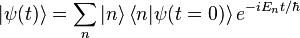Quantum Mechanics
In quantum mechanics, a state of a system is described by a wavefunction which solves the Schrödinger equation. The square of the absolute value of, i.e.
is the probability density to measure the particle in place x at time t.
Usually, when involving some sort of potential, the wavefunction is decomposed into a superposition of energy eigenstates, each oscillating with frequency of . Thus, we may write
The eigenstates have a physical meaning further than an orthonormal basis. When the energy of the system is measured, the wavefunction collapses into one of its eigenstates and so the particle wavefunction is described by the pure eigenstate corresponding to the measured energy.
Read more about this topic: Normal Mode
Famous quotes containing the words quantum and/or mechanics:
“But how is one to make a scientist understand that there is something unalterably deranged about differential calculus, quantum theory, or the obscene and so inanely liturgical ordeals of the precession of the equinoxes.”
—Antonin Artaud (1896–1948)
“It is only the impossible that is possible for God. He has given over the possible to the mechanics of matter and the autonomy of his creatures.”
—Simone Weil (1909–1943)